Drawing Orbital Diagrams
Which are the orbitals(s,p,d,f) have center of symmetry? Orbital diagrams — overview & examples Drawing atomic and molecular orbitals diagrams for molecules
Drawing Atomic and Molecular Orbitals Diagrams for Molecules - Organic
How’re the orbitals in an atom arranged? Orbital overview sulfur caroline monahan How to draw a molecular orbital diagram
Atomic orbitals
Orbitals hybridization hybrid chemistry orbital sp3 atomic hybridized three atoms sp each bond four valence equivalent blue red produces bondingRepresenting atomic orbital configuration electron Orbital diagrams — overview & examplesDrawing atomic and molecular orbitals diagrams for molecules.
Shapes of atomic orbitals — overview & examples6.6: the shapes of atomic orbitals 7.7 orbital shapes and energiesOrbitals shapes atomic chemistry atoms chem quantum electrons shape electron atom numbers wave theory chart cartesian general orbital diagram energy.
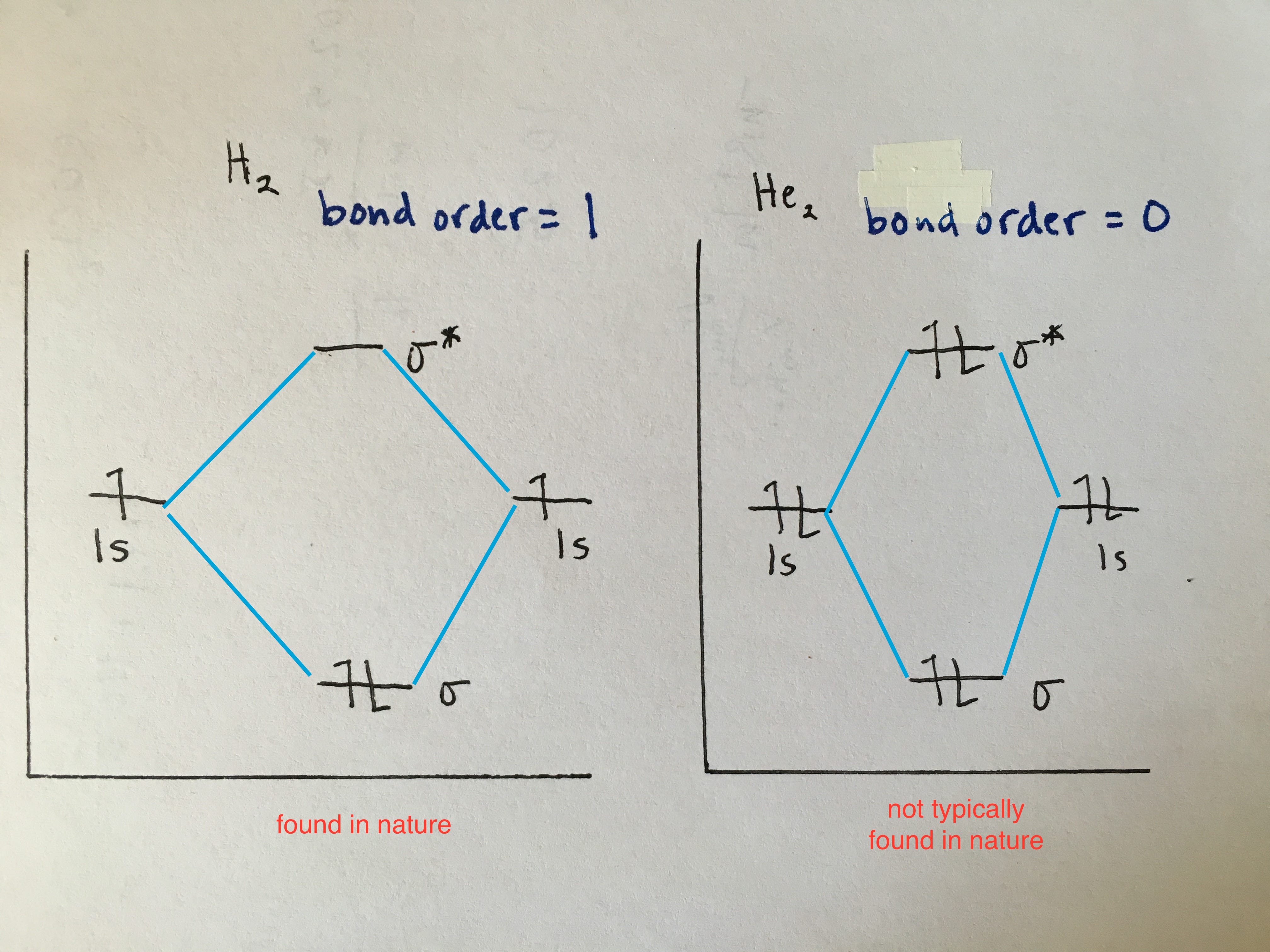
Orbital drawing diagrams
Orbital orbitals electron shapes atomic angular energies single chemwiki diagram electronic nodes radialOrbitals molecular atomic orbital educator diagrams Hybrid atomic orbitalsElectron orbitals electrons quantum chemistry numbers electronic structure introductory orbital model figure atoms number arrangement libretexts text chapter ball energy.
Orbital diagrams monahanHow to do orbital diagrams Orbital orbitals subshell symmetry gerade socraticOrbitals molecular atomic molecules diagrams axis internuclear.

Solved:draw atomic orbital diagrams representing the ground-state
Chapter 8 section b quantum numbers for electronsMolecular orbital diagrams simplified Molecular orbitals atomic orbital molecules socratic mo laidOrbital molecular simplified.
Atom orbitals arrangedUse the molecular orbital diagram shown to determine which of the Orbital molecular hf diagrams megan simplifiedDrawing orbital diagrams.

Orbitals atomic orbital three expii only
Orbital orbitals electron atoms science chemistry britannicaOrbital molecular diagrams determine simplified megan .
.








